Guide to IIoT Analytics
 Download Now
Download NowThe FDA’s guidance on process validation outlines a well accepted set of practices for ensuring that pharmaceutical products are not adulterated. The three stages are rigorous, establishing a set of parameters and spec limits which are baked into the regulatory approvals for the pharmaceutical product, making post-approval changes difficult. This seems to leave little room for introducing new analytics approaches into an existing process. However, this isn’t quite the case.
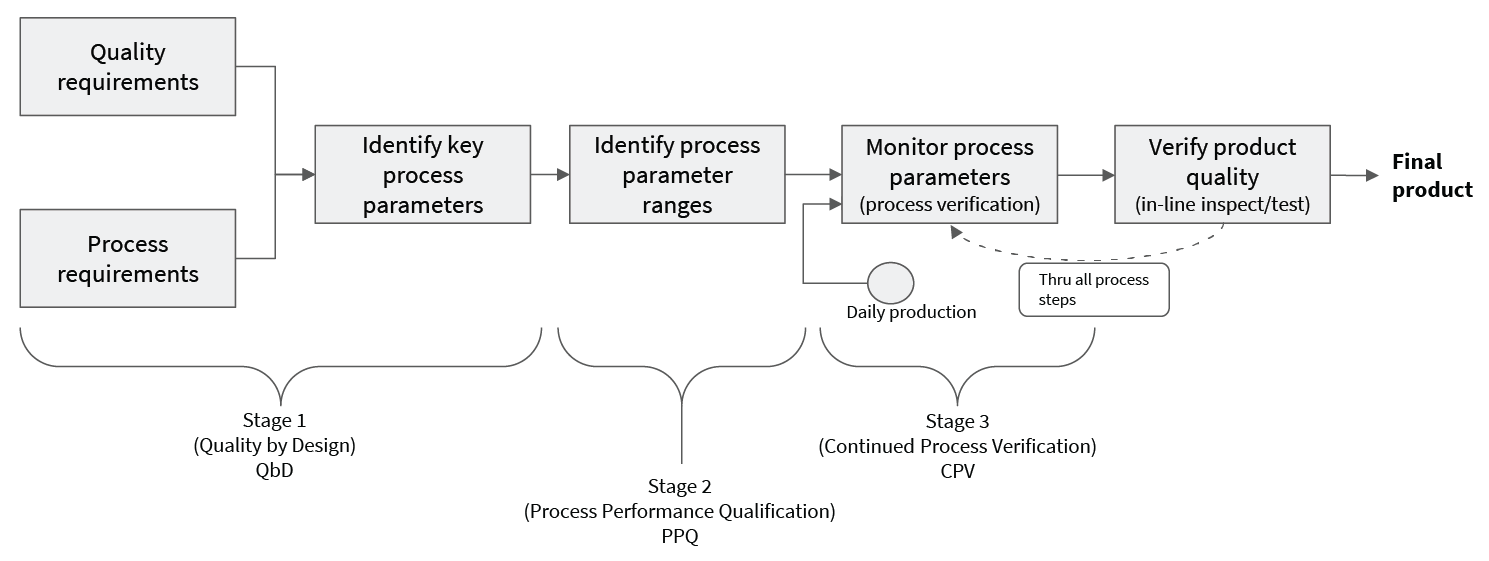
Changing the details of an established Continued Process Verification (CPV) practice is difficult because of the approval process required. However, easily missed is the fact that one can assist the CPV process (see “Regulatory Challenges” pg 39), leaving the regulated portion unchanged. Therefore, inserting analyses that assist CPV carries a much lower burden than might be expected. This leaves significant room for innovation and improvement with very little upfront cost.
One way to think about this is shown in Figs 2 and 3.
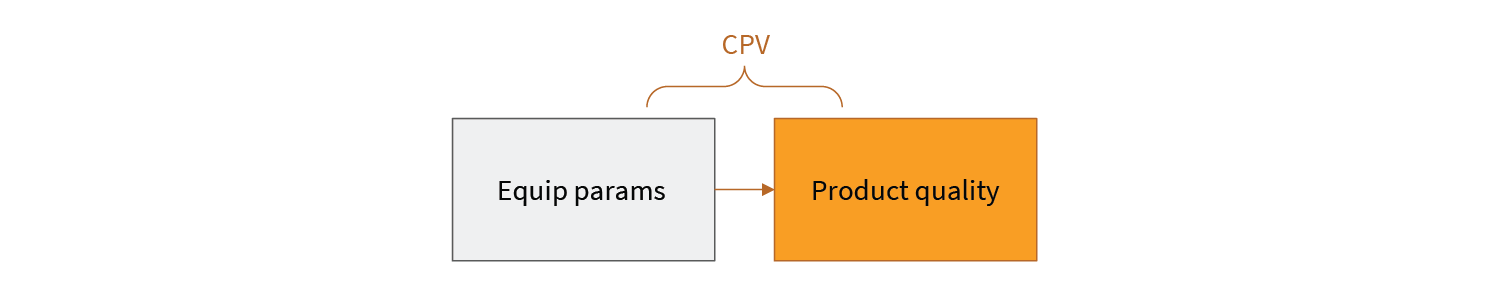
In CPV, equipment parameters are used as a proxy for product quality. Through a thoughtful process of performance qualification, a relationship between equipment behavior (in terms of equipment sensor readings) and process outcomes establishes the bounds of acceptable parameter values. However, there is another relationship that is not as obvious. This is shown in Fig 3.
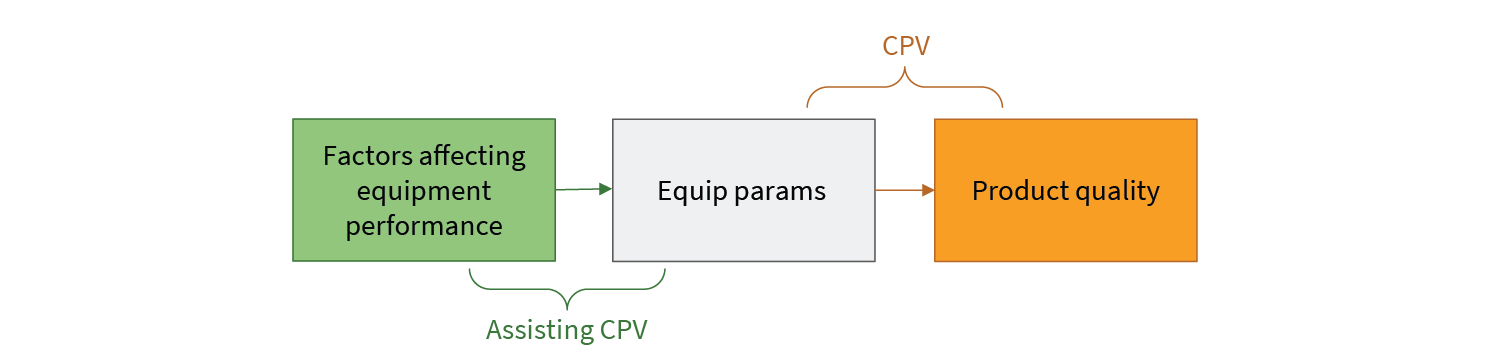
Equipment performance is affected by a number of things such as:
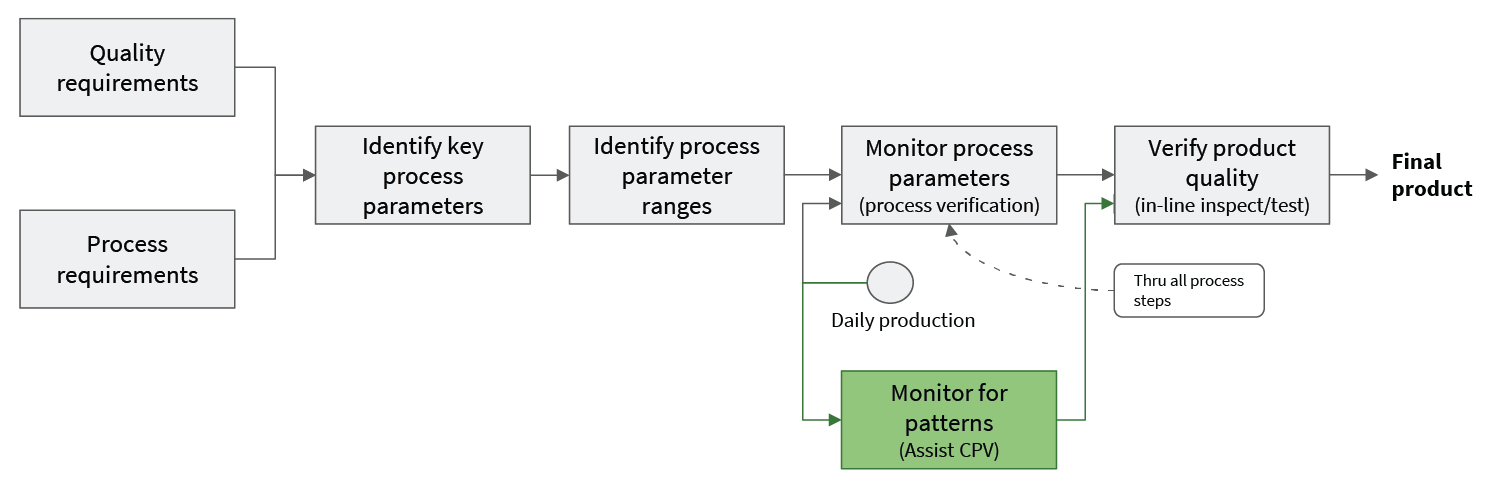
Patterns of behavior reflected in the data from equipment sensors can give insight into these performance affecting factors. In many cases, these patterns develop before product quality is significantly affected. Putting in place analytics that can detect these patterns gives the plant operations team actionable warning before CPV limits indicate a problem. This warning can be used to limit costly production impacts. Importantly, because the CPV process itself is untouched, these kinds of pattern detection analytics can be implemented without additional filings or regulatory delay. Assisting CPV does not mean replacing or even changing CPV (see Fig 4).
Falkonry’s Time Series AI platform is built for this kind of application. Our vendor-agnostic technology plugs easily into existing data pipelines for quick start up. Our focus on making AI easy to use enables the operations teams you already have to find important patterns and to scale up quickly because no data scientists are needed to develop or deploy predictive models. Contact us to discuss where predictive analytics can be of most assistance to you.