Implementing Reliability 4.0 in Pharma
Key takeaways:
- Reliability 4.0 enables a holistic operational control and maintenance strategy
- Applying a Reliability 4.0 approach for critical equipment and processes can pave the path for autonomous asset function, reliability and maintenance
Operational excellence comes from the ability to discover insights that affect production, capture knowledge from those insights, and generate remedies and standard operating procedures thereby improving production. A step above operational excellence, is predictive operational excellence where the aim is to systematically prevent adverse events before they impact operations by exploiting the digital exhaust of operations. To achieve predictive operational excellence outcomes, it is critical to apply AI.
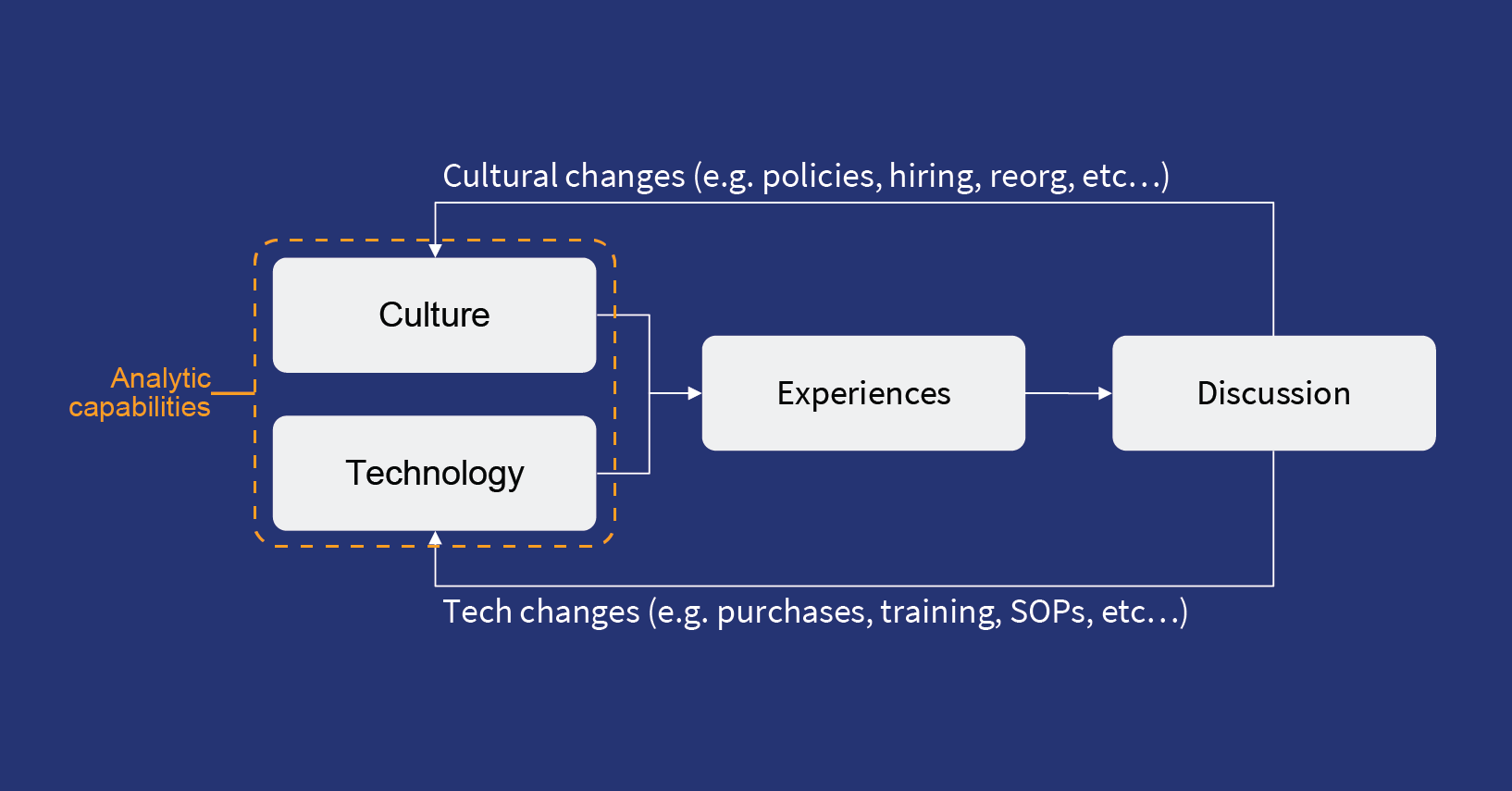
In today’s world of Industry 4.0, it’s time for Reliability 4.0 to come into its own and enable a holistic approach of maintenance, process, and quality. Reliability 4.0 or software-defined reliability offers distinct advantages over traditional post-design reliability practices involving specialized instrumentation, which are often asset-specific. Software-defined reliability makes strategic reliability goals more achievable and forms a key component of a successful long term operational excellence strategy.

An AI platform that enables Reliability 4.0 connects directly with the operational technologies like SCADA, Historians and other systems and utilizes the operational data generated from manufacturing operations to provide incisive insights for vastly superior operational management strategies . Falkonry’s time series AI platform takes the low level, fine grained data and converts it into consumable insights for both the operations and management teams through multivariate pattern analysis and recognition. More importantly, this is done without the need of a data scientist, data engineer or system integrator. Connecting this platform with the existing systems requires minimal effort and this approach can be seamlessly applied across plants and processes for enterprise scale.
Let’s look at a use case of Reliability 4.0 in lyophilization, a highly critical process in pharma implemented by IMA Life, a leading pharma equipment manufacturer. A freeze dryer collects around 5 billion data points in a year which are severely underutilized. A product like Falkonry Clue is required to analyze trends across different real time parameters of the equipment as measured by pre-installed or add-on sensors to detect and predict anomalous conditions. Operational expertise can now be captured directly against these condition events, thus enabling automatic, timely, and scalable recall to improve operational excellence. Clue caters to operational teams by providing asset level data like process data visualization, and event occurrence frequency for reliability management. It also provides a top level summary to management in terms of model and asset performance. The path ahead is to take a proactive approach to combine expert knowledge and operational data thereby converting the current lyophilization process into autonomous freeze drying.
We recently hosted a webinar on Reliability 4.0 in pharmaceuticals where we discussed how to scale reliability and how to implement Reliability 4.0 to achieve predictive maintenance and operational excellence. We also discussed targeted solutions to solve pharma manufacturing challenges such as:
- Condition-based maintenance: Discover anomalous conditions through pattern analysis, predict when a device will fail and assist in root cause explanation. This allows maintenance teams to fix the issue at the right time
- Real time reject: Run AI on the edge to allow operations teams to reduce the false positive samples of vials at the inspection line after the freeze dryer.
- System-wide reliability: Analyze both continuous process and ad hoc specialized instrumentation measurements across multiple assets and process segments.