A new paradigm of reliability management for pharma industry
Key takeaways:
- The pandemic brought immense pressure on pharma companies to maintain supply of drugs, equipment, delivery systems; and reliability teams are under pressure to establish new approaches to achieve high reliability
- Data is being generated at a staggering rate, but organizations are not able to effectively leverage this data for insights and decision making
- Software-defined reliability provides an intelligence-first approach that utilizes existing data to produce actionable insights
Pharmaceutical manufacturing reliability has never been so important as it is today – and not since WWII has the globe faced the scale and scope of production demand for pharmaceuticals and medical devices. For the foreseeable future, every facet of the pharmaceutical industry is now needed to create drugs, delivery systems, protective equipment, life-sustaining machines, and much much more to battle the world-wide Covid pandemic.
Every component, big or small, in the pharmaceutical manufacturing supply chain – from large-scale sterilization equipment to vaccines and the rubber caps on vaccine vials to intubation tubes – is critical – and we just can’t make them fast enough. Reliability in pharmaceutical manufacturing is needed to meet today’s critical production demands.
Pharmaceutical manufacturing equipment reliability has never been more important.
Given the increased importance, the pharmaceutical industry needs to make a paradigm shift in how they tackle reliability.
Today, organizations are moving towards digital transformation and Industry 4.0 with a growing emphasis on AI, analytics and IoT (as per a survey by IOT-Analytics). The pharmaceutical industry is only in the early stages of this transformation with some companies collecting billions of operational parameter points and others yet to begin the journey. Even those already collecting operational parameters and fully aware of the benefits of predictive operational excellence, are simply not able to put their data to use in the face of today’s overwhelming production demands. This group, producing but not using operational data, tells a cautionary tale about the cost of delay. We have seen organizations delay deployment of advanced reliability management systems in pre-Covid days only to find that when they needed it during Covid, it was too late. Remember – if you need predictive maintenance today, it may already be too late!
Enter stage right: Software-defined reliability
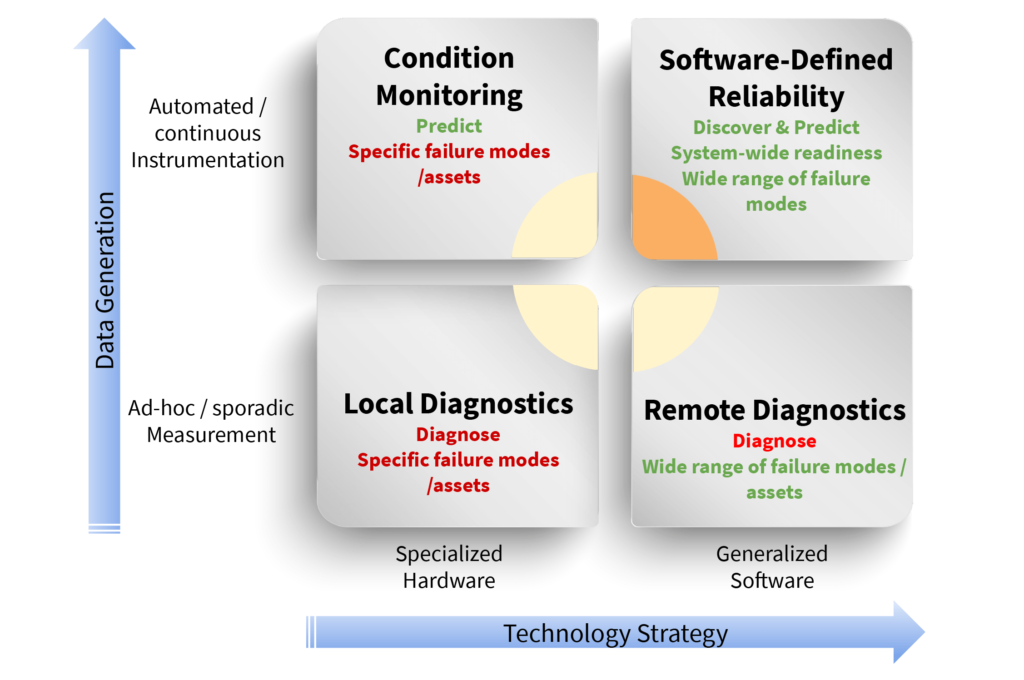
Software-defined reliability through practiced Predictive Production Operations leverages the data that is available “now”. Falkonry’s time series AI helps companies in pharma move from reactive to predictive in a single step using the data they already produce. Instead of running industrial autoclaves, lyophilizers, presses, encapsulators, CIP and SIP skids, or chilling systems to failure then reacting to failures, a predictive strategy can be employed. A predictive production operations strategy mines the parameters being generated by equipment and control systems to discover conditions of operation that precede failure events and predict when those failures may occur.

Software-defined reliability overcomes the shortcomings of asset-specific condition monitoring and remote diagnostics. It analyzes both continuous processes and ad hoc specialized instrumentation measurements. From this operational data, novel behaviors as well as alertable adverse behaviors, are discovered. Asset owners are alerted to these behaviors with sufficient lead time for them to intervene and avoid reliability events.
Early detection of pending equipment malfunction moves pharmaceutical manufacturers from reactive or planned maintenance reliability programs to predictive programs. Leaders in software-defined reliability like IMA Life (Figure 1) are deploying new, ground-breaking, predictive reliability solutions such as SENTINEL LYO built on Falkonry’s Time Series AI Platform.
What does it take to start?
All modern equipment and much of the installed base of manufacturing equipment in pharma and medical device manufacturing are instrumented with OEM-provided sensors, control signals, and alarms. These parameters are usually sufficient for a capable AI to discover and alert operations teams to novel and known operating conditions. Such alerts trigger expert attention and auto-create inspection or work orders in established computerized maintenance management systems (CMMS).
In as little as a couple of days, operating parameters can be streamed to Falkonry’s time series AI platform in real-time. Within a few short weeks, Falkonry learns the expected behavior of monitored entities and begins alerting operations teams of novel and known operating conditions.
When historical data is available and aligned with examples of previous failure behaviors, Falkonry’s AI learns patterns in operating parameters that led up to different kinds of failures. Then, on an ongoing basis, Falkonry will detect and alert operations teams of the onset of these equipment failures with sufficient advance warning to allow them to manage production and maintenance schedules to avoid production losses that would otherwise have been incurred.
Intelligence-first reliability strategy
With an intelligence-first approach to software-defined reliability, you can avoid the “data trap”. Waiting for the “perfect data architecture” or “a better set of sensors” delays the benefits attainable today.
The opportunity cost of not acting today dwarfs the small startup-cost of applying time series AI in a software-defined reliability initiative. Why not start with what you have? Make it work? — then optimize and scale across assets and plants to realize the benefits of software-defined reliability.
Contact us today to learn more about how you can use your operational data to enable predictive operational excellence.




